Participant information sheets are an important aspect for conducting studies within an organization or institution. Information sheets provide potential participants with the necessary details and explanations regarding the procedures of the study.
Apart from this, participant information sheet help to answer any further questions which the participants may have, allowing them to have a clear understanding about the ongoing procedures and to provide them with the required consent.
Participation information sheets are written in an as simple language as possible. They are written to quench the aim of providing sufficient information in a crystal clear manner.
The reason for writing such a sheet in a simplistic manner is to help participants make informed decisions regarding their choice. To make sure that no doubts exist in the minds of participants, institutions approach the creation of the information sheet by explaining the purpose of the research and go on to explain the implications that the research may have on the participants.
Contents
Contents of a Participation Information Sheet
A traditional participation information sheet usually covers the following sections:
- The Title – Usually, the title is written depending on the studies or research being conducted. Institutions, normally try to use a simplified title in such cases when they feel that the original research title is too technical.
- The Invitation – After the title, the invitation paragraph is written. The invitation paragraph provides a brief introduction regarding the project. It makes them aware of the brief purpose for conducting the research and the objectives that it will involve.
Due to this, the invitation paragraph requires all readers to take some time and go through it before they sign up for the study. The invitation paragraph also allows the readers to consult and discuss the paragraph contents with their peers before deciding on whether they want to take part in it or not.
- Purpose – The Purpose section explains the background of study. It explains to the readers the aim of conducting such a study and the duration by which the institution reckons the study to be completed.
- The Reason of Choice – In this section, the participation information sheet explains why the particular person has been chosen for the research.
- A Voluntary Choice – In this section, institutions usually mention the voluntary nature of the project. They make it clear that taking part in the project is completely voluntary and that the participant will not be penalized for refusing from taking part. Also, the section also talks about the fact that users need not worry about any penalization, be it in the present or the distant future.
- The Procedures – The Procedures section explains the procedure of the research or study to the people taking part in the project. Apart from this, the section also mentions other details such as the location of assessments, the number of co-participants, the duration within which the assessments take place and the exact procedures supposed to take place. The procedure section also talks about the details regarding the responsibilities of the participants and what is expected of them during the procedure.
- The Duties of the Participant – The duties section put forward everything that the participant has to do as a part of their duties. Here, institutions make everything clear regarding any restrictions that people might have to go along with if they participate.
- The Possible Advantages – Any benefits or advantages that the participants might be taking away from the research should be mentioned here. The cases in which participants cannot derive any benefits should also be stated clearly. It is not advisable to exaggerate the benefits to the participants during the course of the project as it could be viewed as an act of force.
- The Possible Disadvantages – Similar to the advantages, the participant information sheet should also contain the risks which the person could be a subject to. Any discomforts or drawbacks which the participant could be facing in the duration of the course of study or in future should be clearly mentioned.
- Confidentiality Status – Access to various aspects of information collected from a client during the study require their explicit permission. In this section, the institution conducting the study, explains that the information collected will be kept in firm observation on a completely confidential basis. Apart from this, the section also mentions how the information will be kept ensured.
- The Results – The participants have a right to know the fate of the results of the research. They should be made aware of the whereabouts of their results as well as the details of any person who has access to them. Therefore, the institution should be able to tell the participants if the results are likely to be published and where they can get a copy of their results from.
Apart from this, the institution conducting the study should also include a statement stating details of areas where they might be using the data collected, during the project for additional research purposes.
- Funding Details – This section contains the names and details of the organization/s who are in charge of funding the research.
- Further Details – You should also provide the participant with any further details which they might require. This could be relating to anything which the participants might want to know about the research.
Sample Template for Participation Information Sheet








Creating a sample template for a participation information sheet is not as hard as a lot of people perceive it to be. One can easily create a participation information sheet on the basis template given below:
The Title for The Study
The title produced should be a meaningful one. Try to use titles which can grab the attention of readers by reflecting the contents.
The Invitation and Purpose
Give brief details to the participants about your study. Include the minimum amount of details needed for them to decide if they want to read further into the participant sheet. Next, give details regarding the purpose of the study.
What Would The Study Involve
Explain the contents of the study and what you’re proposing. Explain everything about why you are performing the research and why you’re involving this particular person for the study.
Keep this as simple as you can. Try not to copy directly from some pre-written protocols.
What Would Taking The Part Involve
In this section, mention everything that the participant has to do in accordance with the project. Mention everything about the procedures and the duties of the participant.
What Are The Possible Benefits
Try to elaborate on the possible benefits that the study might have on the participant.
What Are The Possible Drawback
In this section, try to explain the possible disadvantages or implications that the study might have on the person. A few examples which you could include are:
- Impact that the tests might have on people of different age groups
- Impact that the tests might have on your gestation period
- Health-related findings
- The impact which the research may have on your insurance etc.
The Confidentiality Status and The Results
Talk about the confidentiality status surrounding the project and how a person will be provided with their results.
Funding And Further Details
Finally, talk about the funding details. This could consist of the company which has been financing the research and development of this project. Try to provide other details as well. Doing so will help people to contact the firm in the case for any further details of the project.
You can also provide an FAQ section here which can include solutions to questions such as:
- What if the participant wants to discontinue the study in the midst of the programme?
- Who is the person in charge of reviewing the study?
- What happens to any new information which becomes known or is discovered in the midst of the study?
- What happens to the sample generated from the participants?
- Will the study be exploited for any forms of consumer activities, such as advertising?
Checklist To Prepare A Participant Information Sheet







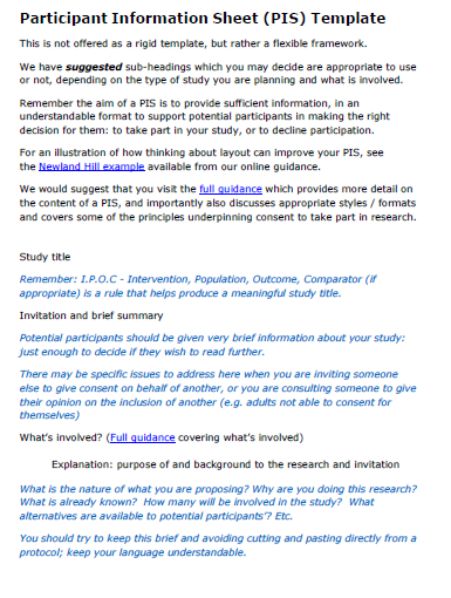
A participant information sheet has several important facets, each of which serves an independent purpose. No two aspects of a participant information sheet are similar to each other in terms of the functions provided by them. Therefore, it is important that each of them are present in the participation information sheet.
Creating a participant information sheet becomes quite easy when it is prepared with the help of a checklist. A checklist is not a complete in-depth study of how a participant information sheet is prepared but it covers all the major topics to be covered.
A few important topics that should be covered in the sheet are:
- First and foremost, the participants should be assured that the entire research project is voluntary and that participants can withdraw at any time that they want.
- Secondly, the participants should be made completely aware of the aims and purposes of the research.
- They should know the purpose of their own participation. The sheet should answer everything relating to the 4 W’s of when, where, who and what.
- The risks should be boldly mentioned. Every inconvenience or discomfort that the participant could expect to contract should be mentioned.
- Bare the complete privacy and confidential information and lay it in front of them. Make them aware of all the facets of the privacy and confidentiality statement.
Taking care of the format of the checklist, you should first divide your sheet into four different rows. The first row will the contain the information, while the other three will function as checkboxes and will be labeled as Yes, No or N/A according to the preference of the person going through the checklist.
Apart from the format, a few other things which you should also keep in mind about the language. These include:
- Write the entire participant information sheet in simple, clear and a non-technical way. It is recommended that you use Plain English.
- Use appropriate language depending on the age group of the targeted audience. Try out different means and choose the one which can be most appropriate.
- Divide the entire text into paragraphs. This helps to make the text easily readable as well as highlight it. Apart from this, it can also help participants to actually understand the written text instead of just skipping over. Also, use as many paragraphs as possible. There are no limits to the number of paragraphs that you can use.
- Use subheading for increased clarity. Apart from this, subheadings also help to give a brief description or introduction of the content matter present in the paragraph present below.
- Before actually bringing the text into circulation, make sure that you can get it proofread by a second pair of eyes. This can help to get rid of any errors that you may have missed. You can also use various software to remove suggestions, suggest better word formations and synonym styles.
Participant Information Sheet for Qualitative Research


Qualitative research is a field of study based on understanding the process of naturalistic inquiry. This field is all about understanding the social occurrences well within their own zone. The field of research focuses on understanding and providing an answer to why instead of what.
A Participant Information Sheet is required as well for studies conducted in the field of qualitative research. Usually, qualitative research is conducted on adults who belong to the non-vulnerable populations are comfortable in dealing with non-sensitive topics. And the participation sheets are fashioned in a manner to suit such needs.
A Participant Information Sheet for Qualitative Research is created similar to traditional sheets. It contains the following sections:
- Who I am and what is this study about?
- What will taking part in the study involve?
- Why have you been invited to take part?
- Is it compulsory for you to take part?
- What are possible disadvantages and drawback which you might encounter?
- What are the possible benefits of the research?
- Is the complete research process confidential?
- How is the information provided by you going to be recorded, stored and protected?
- What will happen to the results?
- Who should be contacted for any further doubts or details?
What is an Informed Consent Form
An informed consent form is a document which is to be mandatorily signed before participants can take part in a clinical research study. Whenever there is a proposal which requires human participants for research, the institution or body conducting the research is required to provide the participants with an informed consent form. In case, the research requires more than one human participant, the consent forms should be provided to each and every participant.
Consent forms are created once the project receives its approval from the ethics review committee of the WHO. These forms are to be mandatorily written in the mother tongue of the subject. For research studies which are performed on a multi-center basis, a consent form is required as the minimum requirement with which participants can take part in research.
A consent form is normally made up of two parts. These are:
- The Information Sheet – The information sheet is tasked with the job of describing the research and the reason for the participant’s involvement with the research.
- The Certificate of Consent – The certificate of consent performs the task of affirming the participant’s consent.
There are a few guidelines which should be followed while writing an informed consent form. These include:
- Similar to the Participant Information Sheet, an Informed Consent Form is also created to be easily readable and understandable by the participants.
- The consent form too should be written in a format which is completely simplistic. All forms of medical terminology should be avoided so that participants going through the form do not get confused with the content mentioned in the form.
- If the research involves a person who is below the age of 18, the consent should be forwarded to a parent or guardian. This is because children below the age of 18 are not considered to be competent in signing a form themselves.
- The consent form should contain the level of confidentiality which will be employed to keep the health information of the participant. Apart from this, the confidentiality clause should also contain details about the people who might have access to the information or details provided by the participant.
- The consent form should also contain the clause that consenting to the research is completely voluntary and that choosing not to participate in the research will not result in any loss of benefits or penalty to the participant.
- Finally, the consent form should contain the contact information which can be used by the participant for enquiring about the details of the study. He/She should also be provided with information regarding any research-related injury or side effect which he might have to face in the midst of the research process.